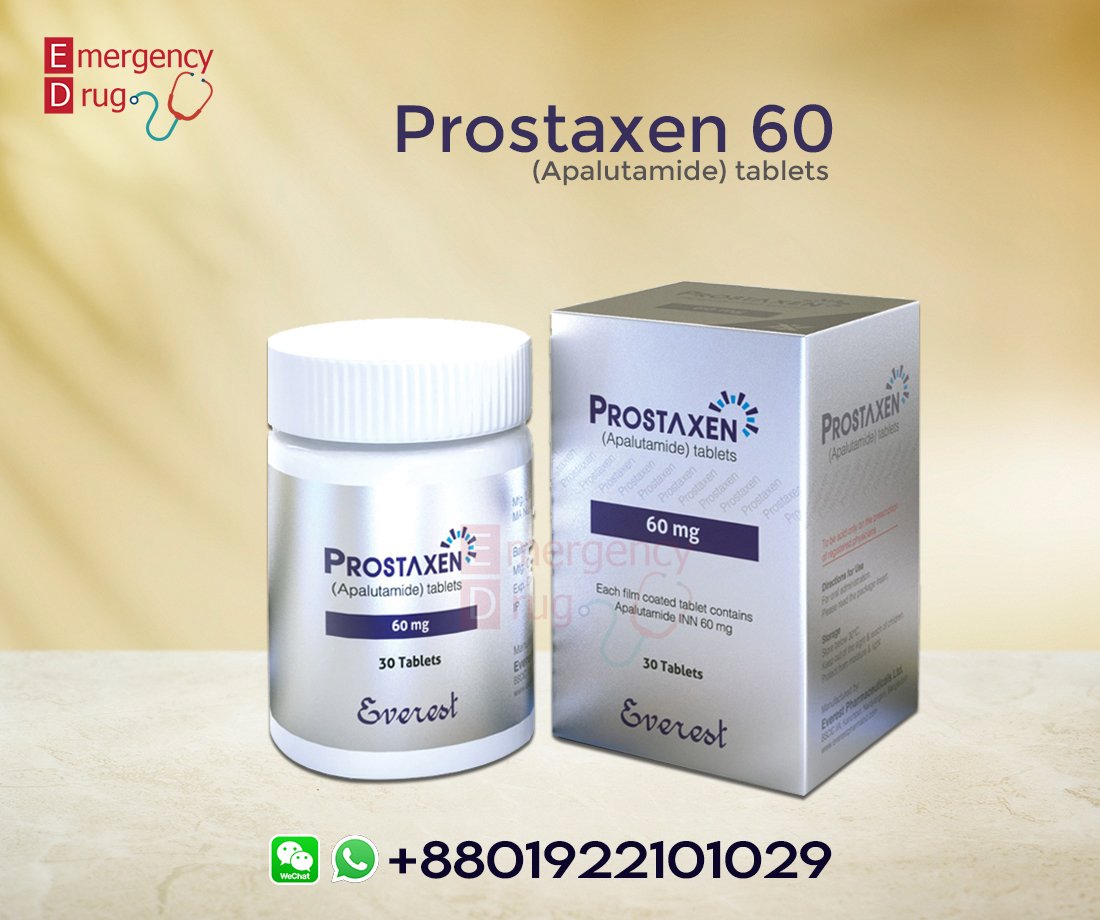
بروستاكسين 60 ملغ (أبالوتاميد) – 30 قرصًا
Price: $145.00
بروستاكسين 60 ملغ (أبالوتاميد) يُستخدم لعلاج أنواع معينة من سرطان البروستاتا (السرطان الذي يصيب الرجال ويبدأ في غدة البروستاتا) والذي انتشر إلى أجزاء أخرى من الجسم أو الذي لم ينتشر إلى أجزاء أخرى من الجسم ولكنه لم يستجب للعلاجات الطبية الأخرى.


Description
مميزات المنتج:
| بروستاكسين | : اسم المنتج |
| أبالوتاميد | : الاسم العام |
| Everest Pharma Ltd | : الشركة المصنعة |
| سرطان البروستات | : إشارة |
| الكمبيوتر اللوحي | : صياغة |
| 60 ملغ | : قوة |
| 30 قرص | : كمية |
| أقل من 30 درجة | : تخزين |
| التصدير فقط | : التسجيلات |
المؤشرات والاستخدام:
يُستخدم أبالوتاميد كمثبط لمستقبلات الأندروجين لعلاج المرضى الذين يعانون من:
- سرطان البروستاتا الحساس للإخصاء المنتشر.
- سرطان البروستاتا المقاوم للإخصاء غير المنتشر.
علم الأدوية:
بروستاكسين 60 ملغ (أبالوتاميد) هو مثبط انتقائي لمستقبلات الأندروجين (AR) يؤخذ عن طريق الفم، حيث يرتبط مباشرة بمجال ارتباط-ligand لمستقبل الأندروجين. يعمل أبالوتاميد على منع انتقال مستقبل الأندروجين إلى النواة، ويثبط ارتباطه بالحمض النووي، ويعيق عملية النسخ المعتمدة على مستقبل الأندروجين، كما أنه لا يمتلك نشاطًا شبيهاً بالأندروجين. يؤدي علاج أبالوتاميد إلى تقليل تكاثر الخلايا السرطانية وزيادة عملية الاستماتة (apoptosis)، مما ينتج عنه نشاط قوي مضاد للأورام.
الجرعة وطريقة الإعطاء:
الجرعة الموصى بها من أبالوتاميد هي 240 ملغ (أربع أقراص بتركيز 60 ملغ) تُؤخذ عن طريق الفم مرة واحدة يوميًا. يُنصح بابتلاع الأقراص كاملة دون سحقها أو مضغها. يمكن تناول أبالوتاميد مع الطعام أو بدونه.
يجب أن يتلقى المرضى أيضًا نظير الهرمون المطلق لموجهة الغدد التناسلية (GnRH) بشكل متزامن أو أن يكونوا قد خضعوا لعملية استئصال الخصيتين الثنائية.
موانع الاستعمال:
- فرط الحساسية للمادة الفعالة.
- النساء الحوامل أو اللاتي قد يصبحن حوامل.
التحذيرات والاحتياطات:
- نوبات الصرع: لا يُوصى باستخدام أبالوتاميد في المرضى الذين لديهم تاريخ من نوبات الصرع أو عوامل مؤهبة أخرى، بما في ذلك – على سبيل المثال لا الحصر – إصابات الدماغ الكامنة، السكتة الدماغية الحديثة (خلال عام واحد)، أورام الدماغ الأولية أو النقائل الدماغية. في حال حدوث نوبة صرع أثناء العلاج بـ أبالوتاميد، يجب التوقف عن العلاج نهائيًا.
- في دراستين عشوائيتين (SPARTAN و TITAN)، حدثت نوبات صرع لدى 0.4% من المرضى الذين تلقوا أبالوتاميد، مقارنة بـ 0.2% في المرضى الذين عولجوا بدواء وهمي. استبعدت هذه الدراسات المرضى الذين لديهم تاريخ من نوبات الصرع أو عوامل مؤهبة لها.
- السقوط والكسور: لوحظت حالات سقوط وكسور لدى المرضى الذين يتلقون أبالوتاميد. لذا، يجب تقييم خطر السقوط والكسور قبل بدء العلاج والاستمرار في مراقبة المرضى وإدارتهم وفقًا للإرشادات العلاجية المعتمدة، كما يجب النظر في استخدام العوامل المستهدفة للعظام.
- مرض القلب الإقفاري: تم تسجيل حالات مرض القلب الإقفاري، بما في ذلك أحداث قاتلة، لدى المرضى الذين عولجوا بـ أبالوتاميد، حيث كان لدى معظم المرضى عوامل خطر قلبية. يجب مراقبة المرضى بحثًا عن علامات وأعراض مرض القلب الإقفاري، كما ينبغي تحسين إدارة عوامل الخطر القلبية الوعائية، مثل ارتفاع ضغط الدم، السكري، أو اضطراب شحوم الدم، وفقًا لمعايير الرعاية المتبعة.
الاستخدام المتزامن مع أدوية أخرى:
يُعد بروستاكسين 60 ملغ محفزًا قويًا للإنزيمات، مما قد يؤدي إلى فقدان فعالية العديد من الأدوية الشائعة الاستخدام. لذلك، ينبغي تجنب الاستخدام المتزامن لـ أبالوتاميد مع الأدوية التي تُعد ركائز حساسة للعديد من الإنزيمات الأيضية أو نواقل الأدوية، خاصة إذا كان تأثيرها العلاجي مهمًا للمريض، ولم يكن من السهل تعديل الجرعة بناءً على مراقبة الفعالية أو تركيز البلازما.
يجب تجنب التناول المشترك لأبالوتاميد مع الوارفارين أو مضادات التخثر الشبيهة بالكومارين. إذا تم تناوله مع مضاد تخثر يُستقلب بواسطة CYP2C9 (مثل الوارفارين أو الأسينوكومارول)، فيجب إجراء مراقبة إضافية لنسبة التطبيع الدولية (INR).
الأمراض القلبية الوعائية الحديثة:
استُبعد المرضى الذين يعانون من أمراض قلبية وعائية سريرية هامة خلال الأشهر الستة الماضية من الدراسات السريرية، بما في ذلك:
- الذبحة الصدرية الشديدة أو غير المستقرة.
- احتشاء عضلة القلب.
- فشل القلب الاحتقاني المصحوب بأعراض.
- أحداث الخثار الشرياني أو الوريدي (مثل الانصمام الرئوي أو السكتة الدماغية، بما في ذلك النوبات الإقفارية العابرة).
- اضطرابات عدم انتظام ضربات القلب البطينية ذات الأهمية السريرية.
لذلك، لم يتم إثبات أمان أبالوتاميد في هؤلاء المرضى. وإذا تم وصف أبالوتاميد، فيجب مراقبة المرضى الذين يعانون من أمراض القلب والأوعية الدموية بشكل دقيق، مع متابعة عوامل الخطر مثل فرط كوليسترول الدم، فرط ثلاثي الغليسريد، أو اضطرابات القلب الأيضية الأخرى، ومعالجتها وفقًا للإرشادات العلاجية المعتمدة.
العلاج بحرمان الأندروجين قد يطيل فترة QT:
في المرضى الذين لديهم تاريخ من إطالة فترة QT أو عوامل خطر مرتبطة بها، وكذلك في المرضى الذين يتناولون أدوية أخرى قد تطيل فترة QT، يجب على الأطباء تقييم نسبة الفائدة إلى المخاطر، بما في ذلك احتمالية الإصابة باضطراب تورساد دي بوانت (Torsade de Pointes)، قبل بدء العلاج بـ أبالوتاميد.
الآثار الجانبية:
تمت ملاحظة التفاعلات الضارة التالية ذات الأهمية السريرية في التجارب السريرية:
- اضطرابات الغدد الصماء: قصور الغدة الدرقية.
- اضطرابات التمثيل الغذائي والتغذية: فرط كوليسترول الدم، فرط ثلاثي الغليسريد.
- اضطرابات الجهاز العصبي: خلل التذوق، نوبة صرع.
- اضطرابات القلب: مرض القلب الإقفاري.
- اضطرابات الأوعية الدموية: الهبات الساخنة، ارتفاع ضغط الدم.
- اضطرابات الجهاز الهضمي: الإسهال.
- اضطرابات الجلد والأنسجة تحت الجلد: طفح جلدي، حكة.
- اضطرابات الجهاز العضلي الهيكلي والنسيج الضام: الكسور، ألم المفاصل، تشنج العضلات.
- اضطرابات عامة وظروف موقع الإعطاء: التعب.
- الفحوصات: انخفاض الوزن.
- إصابات وتسمم ومضاعفات الإجراءات الطبية: السقوط، الكسر.
التداخلات الدوائية:
تأثير الأدوية الأخرى على أبالوتاميد:
- CYP2C8 يلعب دورًا في التخلص من أبالوتاميد وتشكيل مستقلبه النشط. في دراسة تفاعل دوائي، انخفضت Cmax لأبالوتاميد بنسبة 21% بينما زادت AUC بنسبة 68% عند التناول المشترك لـ أبالوتاميد 240 ملغ لمرة واحدة مع جيمفيبروزيل (مثبط قوي لـ CYP2C8). بالنسبة للمواد النشطة (مجموع أبالوتاميد ومستقلبه النشط المعدل بالفعالية)، انخفضت Cmax بنسبة 21% بينما زادت AUC بنسبة 45%.
- لا يلزم تعديل الجرعة الأولية عند استخدام أبالوتاميد مع مثبط قوي لـ CYP2C8 (مثل جيمفيبروزيل أو كلوبيدوغريل)، لكن يجب مراعاة تقليل الجرعة بناءً على تحمل المريض للعلاج.
- المثبطات الخفيفة أو المتوسطة لـ CYP2C8 ليست متوقعة أن تؤثر على تعرض أبالوتاميد.
- CYP3A4 يلعب أيضًا دورًا في التخلص من أبالوتاميد وتكوين مستقلبه النشط. في دراسة تفاعل دوائي، انخفضت Cmax لأبالوتاميد بنسبة 22% بينما بقيت AUC ثابتة عند التناول المشترك لـ أبالوتاميد 240 ملغ لمرة واحدة مع إيتراكونازول (مثبط قوي لـ CYP3A4). بالنسبة للمواد النشطة، انخفضت Cmax بنسبة 22% بينما بقيت AUC ثابتة.
- لا يلزم تعديل الجرعة الأولية عند استخدام أبالوتاميد مع مثبط قوي لـ CYP3A4 (مثل كيتوكونازول، ريتونافير، كلاريثروميسين)، لكن يجب مراعاة تقليل الجرعة بناءً على تحمل المريض للعلاج.
- المثبطات الخفيفة أو المتوسطة لـ CYP3A4 ليست متوقعة أن تؤثر على تعرض أبالوتاميد.
- لم يتم تقييم تأثير محفزات CYP3A4 أو CYP2C8 على الحرائك الدوائية لأبالوتاميد في الدراسات السريرية، ولكن من غير المتوقع أن يكون لها تأثير سريري ملحوظ على تركيز أبالوتاميد. لذلك لا يلزم تعديل الجرعة عند الاستخدام المتزامن مع محفزات CYP3A4 أو CYP2C8.
تأثير أبالوتاميد على الأدوية الأخرى:
- ركائز CYP3A4، CYP2C9، CYP2C19 و UGT:
- أبالوتاميد هو محفز قوي لـ CYP3A4 و CYP2C19، و محفز ضعيف لـ CYP2C9 في البشر.
- الاستخدام المشترك لـ أبالوتاميد مع الأدوية التي يتم استقلابها بشكل أساسي بواسطة CYP3A4 أو CYP2C19 أو CYP2C9 يمكن أن يؤدي إلى انخفاض تعرض هذه الأدوية. من المستحسن استبدال هذه الأدوية إذا كان ذلك ممكنًا، أو مراقبة فقدان الفعالية إذا استمرت الأدوية المستخدمة.
- التناول المشترك لـ أبالوتاميد مع الأدوية التي هي ركائز لـ UDP-glucuronosyl transferase (UGT) يمكن أن يؤدي إلى انخفاض التعرض لهذه الأدوية. يجب توخي الحذر إذا كان يجب التناول المشترك مع ركائز UGT ومراقبة فقدان الفعالية.
- ركائز P-gp، BCRP أو OATP1B1:
- تم إظهار أن أبالوتاميد هو محفز ضعيف لـ P-glycoprotein (P-gp) و bcrp (بروتين مقاومة سرطان الثدي) و OATP1B1 سريريًا. عند التوازن الثابت، أبالوتاميد يقلل من التعرض البلازمي لـ فكسوفينادين (ركيزة P-gp) و روزوفاستاتين (ركيزة BCRP/OATP1B1).
- الاستخدام المشترك لـ أبالوتاميد مع الأدوية التي هي ركائز لـ P-gp أو BCRP أو OATP1B1 يمكن أن يؤدي إلى انخفاض التعرض لهذه الأدوية.
- يجب توخي الحذر إذا كان من الضروري تناول الأدوية التي هي ركائز لـ P-gp أو BCRP أو OATP1B1 مع أبالوتاميد ومراقبة فقدان الفعالية إذا استمرت الأدوية.
استخدام أبالوتاميد في الفئات السكانية الخاصة:
- الحمل:
يُمنع استخدام أبالوتاميد في النساء الحوامل أو اللاتي قد يُصبحن حوامل. بناءً على آلية عمله، قد يسبب أبالوتاميد أضرارًا قاتلة إذا تم تناوله أثناء الحمل. لا توجد بيانات متاحة من استخدام أبالوتاميد في النساء الحوامل. لم يتم إجراء دراسات على الإنجاب في الحيوانات باستخدام أبالوتاميد. - الخصوبة:
بناءً على الدراسات الحيوانية، قد يقلل أبالوتاميد من الخصوبة لدى الذكور ذوي القدرة على الإنجاب. - الرضاعة الطبيعية:
لا يُعرف ما إذا كان أبالوتاميد أو مستقلباته يتم إفرازها في حليب الأم. لا يمكن استبعاد الخطر على الطفل الرضيع. يجب عدم استخدام أبالوتاميد أثناء الرضاعة الطبيعية. - الذكور والإناث من ذوي القدرة الإنجابية:
- وسائل منع الحمل في الذكور والإناث:
لا يُعرف ما إذا كان أبالوتاميد أو مستقلباته موجودة في السائل المنوي. قد يكون أبالوتاميد ضارًا للجنين النامي. بالنسبة للمرضى الذين يمارسون الجنس مع شركاء إناث ذوي القدرة الإنجابية، يجب استخدام واقي ذكري مع طريقة أخرى فعالة جدًا لمنع الحمل أثناء العلاج ولمدة 3 أشهر بعد الجرعة الأخيرة من أبالوتاميد.
- وسائل منع الحمل في الذكور والإناث:
التسمم:
لا يوجد ترياق محدد معروف للتسمم بـ أبالوتاميد. في حال حدوث التسمم، يجب التوقف عن استخدام أبالوتاميد واتخاذ التدابير الداعمة العامة حتى يتم تقليل التسمم السريري أو حله.
كيفية التوريد:
أقراص بروستاكسين: يحتوي كل حاوية من بروستاكسين على 30 قرصًا مغلفًا بالفيلم (كل قرص يحتوي على 60 ملغ من أبالوتاميد)، مع مادة تجفيف هلام السيليكا وملف بوليستر مع غطاء مقاوم للأطفال.
No images in this gallery yet!
Related products
-

بروستاكسين 60 ملغ (أبالوتاميد) – 30 قرصًا
Price: $145.00








Reviews
There are no reviews yet.