Description
Xevirol (Everolimus) indicate in advanced Hormone Receptor-Positive, HER2-Negative Breast Cancer (Advanced HR+ BC), Advanced Neuroendocrine Tumors (NET); Advanced Renal Cell Carcinoma (RCC); Renal Angiomyolipoma With Tuberous Sclerosis Complex (TSC); Subependymal Giant Cell Astrocytoma (SEGA) With Tuberous Sclerosis Complex (TSC)
Product Features:
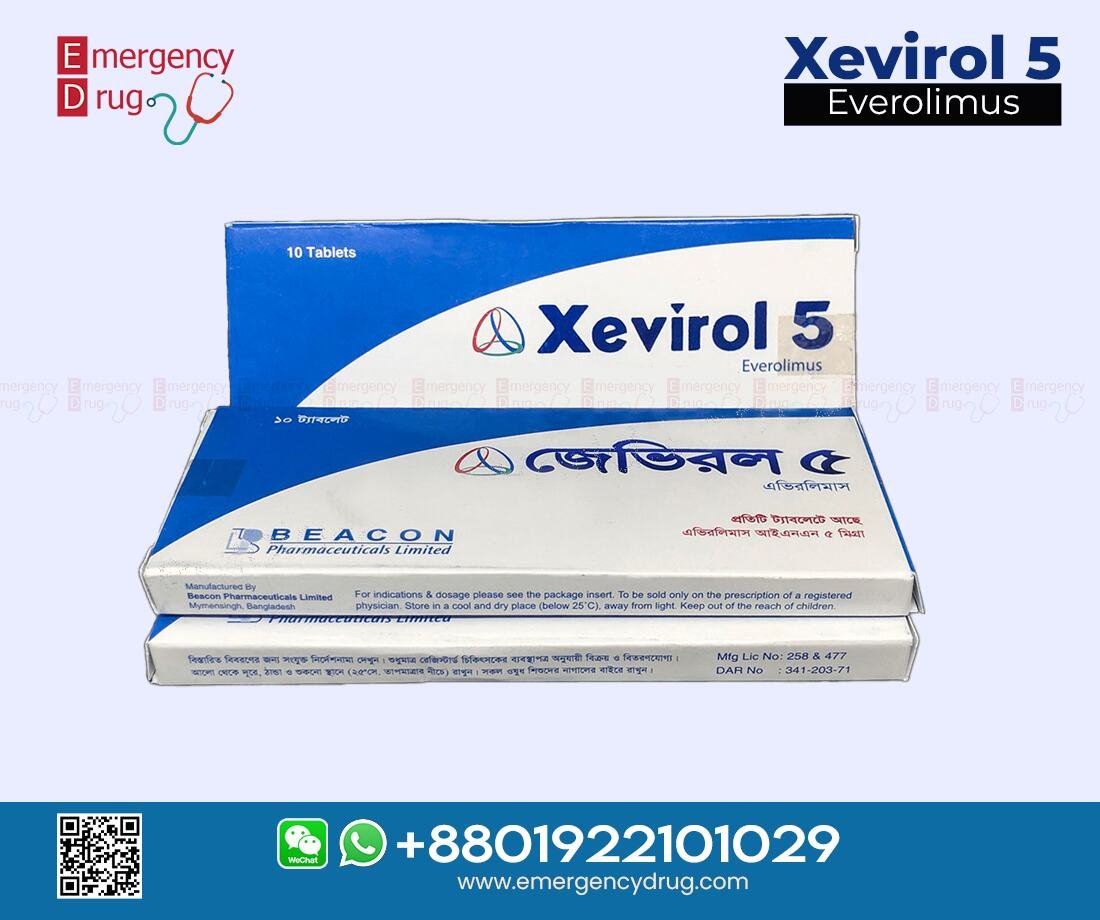
| Product Name | : Xevirol |
| Generic Name | : Everolimus |
| Manufacturer | : Beacon Pharma Ltd |
| Indication | : Advanced Renal Cell Carcinoma |
| Formulation | : Tablet |
| Strength | : 5 mg |
| Quantity | : 10 Tablets |
| Storage | : Room temperature |
| Registrations | : Export Only |
In combination with ciclosporin for microemulsion & corticosteroids for the prophylaxis of organ rejection in adult patients at low to moderate immunological risk receiving an allogeneic renal or cardiac transplant. In combination with tacrolimus & corticosteroids for the prophylaxis of organ rejection in patients receiving hepatic transplant.
Dosage & Administration
General kidney & heart transplant population: Initially 0.75 mg bd administered as soon as possible after transplantation.
Hepatic transplant: 1 mg bd with initial dose starting 4 wk after transplantation.
Advanced Hormone Receptor-Positive, HER2-Negative Breast Cancer, Advanced NET, Advanced RCC, And Renal Angiomyolipoma With TSC: The recommended dose is 10 mg, to be taken once daily at the same time every day. Administer either consistently with food or consistently without food. Everolimus Tablets should swallowed whole with a glass of water. Do not break or crush tablets. Continue treatment until disease progression or unacceptable toxicity occurs.
Interaction
CYP3A4 inhibitors &/or inducers, CYP2D6 substrates with narrow therapeutic index, PgP inhibitors, rifampicin, ACE inhibitors, grapefruit juice & live vaccines.
Contraindications
Patients with hypersensitivity to the active substance, to other rapamycin derivatives, or to any of the excipients. Hypersensitivity reactions manifested by symptoms including, but not limited to, anaphylaxis, dyspnea, flushing, chest pain, or angioedema (e.g., swelling of the airways or tongue, with or without respiratory impairment) have been observed with everolimus and other rapamycin derivatives.
Side Effects
Stomatitis, Constipation, Infections, Asthenia, Fatigue, Cough, Diarrhea, Rash, Anemia, Nausea, Anorexia, Edema, peripheral, Dyspnea, Pyrexia, Vomiting, Headache, Epistaxis, Decreased lymphocytes, Grade 3, Increased glucose, Grade 3, Pneumonitis, Pruritus, Dry skin, Decreased Hgb, Grade 3, Menstrual irregularities, Dysgeusia, Hypertension, Hemorrhage, Tachycardia, CHF.
Pregnancy & Lactation
Pregnancy Category D. There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks.
Lactation: Distribution into breast milk is unknown; not recommended.
Precautions & Warnings
Patients taking concomitant ACE inhibitor therapy may at increase risk for angioedema. Interstitial lung disease/noninfectious pneumonitis; monitor for clinical symptoms or radiological changes; fatal cases have occurred; manage by dose reduction or discontinuation until symptoms resolve, and consider use of corticosteroids; pulmonary hypertension (including pulmonary arterial hypertension) as a secondary event reported
Elicits immunosuppressive effects and may increase risk for infections; some infections have been severe or fatal; monitor for signs and symptoms and treat promptly. Pneumocystis jiroveci pneumonia, some with a fatal outcome, reported; this may be associated with concomitant use of corticosteroids or other immunosuppressive agents
Mouth ulcers, stomatitis, and oral mucositis are common; management includes mouthwashes (without alcohol or peroxide) and topical treatments May delay wound healing and increase wound-related complications (eg, dehiscence, wound infection, incisional hernia, lymphocele, and seroma)
- Cases of renal failure (including acute renal failure), some fatal observe.
- May cause angioedema and fluid accumulation
- Decreases Hgb, lymphocytes, ANC, platelets; increases cholesterol, TG, glucose, creatinine
- Elevations of serum creatinine, urinary protein, blood glucose, and lipids may occur;
- decreases in hemoglobin, neutrophils, and platelets may also occur; monitor renal
- function, blood glucose, lipids, and hematologic parameters prior to treatment and periodically thereafter
- May impair male fertility
- Child-Pugh Class C hepatic impairment
- Avoid use of live vaccines during treatment and close contact with live vaccine recipients
- Can cause fetal harm when administered to a pregnant woman; advise female patients of reproductive potential to use highly effective contraception while receiving everolimus and for up to 8 weeks after ending treatment.
Visit our another product here:




Reviews
There are no reviews yet.