Tucatinib
Showing the single result
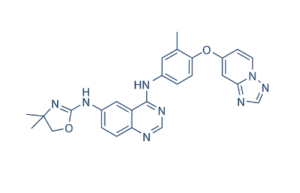
Tucatinib Chemical Structure
What is Tucatinib Drug
Tucatinib drug used to treat HER2-positive breast cancer and colorectal cancer. It is an oral tyrosine kinase inhibitor that specifically targets the HER2 protein, helping to inhibit the growth of cancer cells. Tucatinib is often used in combination with other cancer treatments, such as trastuzumab and capecitabine.
Breast Cancer
Breast cancer is caused by the uncontrolled growth of abnormal cells in the breast tissue, which can invade nearby tissues and, in advanced stages, metastasize to other parts of the body via the lymphatic system or bloodstream. Although the exact cause of breast cancer is not fully
Colorectal Cancer
Colorectal cancer (CRC) is a common form of cancer that originates in the colon or rectum. It affects both men and women and ranks as one of the most common and deadly cancers worldwide. The disease often begins with benign polyps, which can turn into malignant tumors if left untreated. Early detection and prompt treatment through screening tests such as colonoscopy are crucial to improving outcomes and reducing mortality associated with CRC.
olonoscopy are crucial to improving outcomes and reducing mortality associated with CRC.
Medication of Tucatanib
Tucatinib is a drug classified as an oral tyrosine kinase inhibitor. It specifically targets the HER2 protein, which is overexpressed in some breast cancers. Tucatanib in combination with trastuzumab and capecitabine is indicated for the treatment of patients with advanced unresectable or metastatic HER2-positive breast cancer, including brain metastases, who have received one or more prior anti-HER2-based regimens for metastatic disease.
Tucatinib Indication
Tucatanib is indicated for the treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer. It is specifically used in combination with trastuzumab and capecitabine for patients who have previously undergone one or more anti-HER2-based therapies in the metastatic setting, including brain metastases.
Pharmacology
Tucatinib is a tyrosine kinase inhibitor of HER2. In vitro, tucatinib inhibits the phosphorylation of HER2 and HER3, thereby inhibiting downstream MAPK and AKT signaling and cell proliferation, and shows anti-tumor activity in HER2-expressing tumor cells. In vivo, tucatinib inhibits the growth of HER2-expressing tumors. The combination of tucatinab and trastuzumab increased anti-tumor activity in vitro and in vivo compared to either drug alone.
Cardiac Electrophysiology:
Treatment with tucatinib at the recommended dose of 300 mg orally twice daily did not increase QTc (i.e., > 20 ms).
Pharmacokinetics:
Tucatinib showed proportional increases in AUC0-INF and Cmax over a dose range of 50 mg to 300 mg (0.17 to 1 times the approved recommended dose). After tucatanib 300 mg twice daily for 14 days, it accumulated with a 1.7-fold increase in AUC and a 1.5-fold increase in Cmax. A steady state is reached approximately 4 days after starting tucatinib dosing.
Absorption:
After oral administration, Tucatanib is rapidly absorbed into the bloodstream. It reaches peak plasma concentrations in about 2 hours (range 1 to 4 hours).
Distribution:
At clinically relevant concentrations, tucatinib is 97.1% bound to plasma proteins. It exhibits a moderate volume distribution, with a geometric mean apparent volume of approximately 1670 L (66%), indicating its distribution in tissues outside the bloodstream.
Elimination:
Tucatinib is primarily eliminated through the feces, with small amounts excreted in the urine. It has a geometric mean half-life of approximately 8.5 hours (CV 21%) and an apparent clearance of 148 L/h (55%).
Metabolism:
The liver metabolizes tucatinib primarily via CYP3A4, with CYP2C8 contributing to a lesser extent.
Excretion:
Following a single oral dose of 300 mg of radiolabeled tucatinib, the body recovers 4.1% of the total radiolabeled dose in feces (including 16% of the dose as unchanged tucatanib) and urine, achieving overall recovery. 90% within 13 days. In postdose plasma, approximately 76% of plasma radioactivity consisted of unchanged tucatanib, while 19% was attributed to identified metabolites, and approximately 5% was unchanged.
HER2 Inhibition:
Tucatinib blocks HER2 kinase activity, leading to decreased phosphorylation and subsequent inhibition of downstream signaling pathways.
Cellular Effects:
By inhibiting HER2 signaling, tucatinib stops cancer cell proliferation and induces apoptosis in HER2-positive breast cancer cells.
Dosage & Administration
The recommended dose of tucatinib is 300 mg orally twice daily, with trastuzumab and capecitabine, until disease progression or unacceptable toxicity occurs. Patients should swallow tucatinib tablets whole and avoid chewing, crushing, or splitting before swallowing. Tablets should not be taken if broken, cracked, or otherwise damaged.
Patients instructed to take Tucatinib approximately 12 hours apart and at the same time consistently, with or without food. In case a dose is missed or vomited, patients should take the next dose at the regularly scheduled time.
When used with tucatinib, the recommended dose of capecitabine is 1000 mg/m2 orally twice daily, 30 minutes before meals. Tucatanib and capecitabine can be taken at the same time.
Overdose Effects
The effects of tucatinib overdose have not specifically reported. However, in case of overdose, treatment with Tucatinib tablets should be stopped and general supportive measures should be applied.
Miss dose Effects
Missing a dose of tucatinib may affect the effectiveness of the treatment. So if a dose of Tucatinib is missed, consult your doctor immediately.
Use in Special Populations
Pregnancy:
Tucatinib may cause fetal harm when administered to a pregnant woman. There is no human data on the use of Tucatinib in pregnant women to determine drug-related risks. Advise pregnant women and women of childbearing potential of the potential risk to the fetus. The background risk of major birth defects and miscarriage in the indicated population remains unknown.
Lactation:
There are no data on the presence of Tucatinib or its metabolites in human or animal milk, nor are there any data on its effects on breastfed infants or milk production. Because of the potential for serious adverse reactions in a nursing infant, women are advised not to breastfeed during treatment with Tucatinib and for at least 1 week after the end of dosing.
Pregnancy Testing:
Women of childbearing potential should be tested for pregnancy before starting treatment with Tucatinib.
Contraception Females:
Women of reproductive potential should use effective contraception during treatment with Tucatanib and for at least one week after the last dose.
Contraception Males:
Male patients with female partners of reproductive potential should use effective contraception during treatment with Tucatanib and for at least one week after dosing.
Infertility:
Based on animal studies, tucatinib may impair fertility in both males and females.
Pediatric Use:
The safety and efficacy of Tucatinib in pediatric patients have not been established.
Geriatric Use:
In the HER2CLIMB trial, 82 patients who received tucatanib were 65 years of age or older, including 8 patients who were 75 years of age or older. The incidence of serious adverse reactions was 34% in patients 65 years of age or older, compared with 24% in patients younger than 65 years. The most frequent serious adverse reactions in patients 65 years of age and older who received tucatanib were diarrhea (9%), vomiting (6%), and nausea (5%). No overall difference in the efficacy of tucatinib was observed between patients 65 years of age or older and younger patients. The number of patients aged 75 years and older was too small to determine differences in efficacy or safety.
Side Effects of tucatinib 150 mg
The following Clinically significant side effects include:
- Diarrhea
- Hepatotoxicity
The most frequently reported adverse reactions in patients receiving tucatinib (>20%) were diarrhea, palmar-plantar erythrodysesthesia(PPE) syndrome, nausea, fatigue, hepatotoxicity, vomiting, stomatitis, loss of appetite, abdominal pain, headache, anemia, and rash.
Precautions & Warnings
Diarrhea:
Tucatinib can cause severe diarrhea, leading to dehydration, hypotension, acute kidney injury, and death. In the HER2CLIMB trial, 81% of patients who received tucatinib developed diarrhea, 12% grade 3 diarrhea, and 0.5% grade 4 diarrhea. Both patients with grade 4 diarrhea subsequently died, with diarrhea contributing to their deaths. The median time to onset of the first episode of diarrhea was 12 days, and the median time to resolution was 8 days. Tucatinib dose was reduced in 6% of patients due to diarrhea and discontinued in 1% of patients.
Prophylactic use of antidiarrheal treatment was not required in the HER2CLIMB trial. If diarrhea occurs, administer antidiarrheal therapy as clinically indicated. Perform necessary diagnostic tests to rule out other causes of diarrhea. Based on the severity of diarrhea, interrupt the dose, then either reduce the dose or permanently discontinue Tucatinib.
Hepatotoxicity:
Tucatinib use may cause severe hepatotoxicity. In HER2CLIMB, 8% of patients who received tucatinib had ALT elevations >5 times the upper limit of normal (ULN), 6% had AST elevations >5 times ULN, and 1.5% had bilirubin elevations >3. Bar ULN (grade) >3. ) hepatotoxicity led to dose reduction of tucatinib in 8% of patients and discontinuation in 1.5% of patients.
Monitor ALT, AST, and bilirubin levels before starting tucatinib, every 3 weeks during treatment, and as clinically indicated. Depending on the severity of hepatotoxicity, interrupt the dose, then either reduce the dose or permanently discontinue Tucatinib.
Therapeutic Class
The therapeutic class of tucatinib is a kinase inhibitor, specifically targeting HER2 (human epidermal growth factor receptor 2).
FDA approval
Tucatinib is an oral tyrosine kinase inhibitor that targets the HER2 protein, which is overexpressed in some breast cancers. The US Food and Drug Administration (FDA) approved tucatinib in April 2020 for the treatment of advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases. The FDA granted this approval based on the results of the HER2CLIMB trial, which showed that tucatinib, trastuzumab, and capecitabine significantly improved patients’ progression-free survival and overall survival.
Storage Conditions
Do not store above 25°C. And keep it in a cool and dry place. keep away from light. Keep out of reach of children.
The Brand name of the Tucatinib Generic
The brand name is TUKYSA. It is an oral medication use to treat breast cancer and colorectal cancer. Tukysa is a very expensive dose. For a 60-tablet supply of the 150 mg dosage, Tucatinib (Tukysa) price is around $13,504, and for the 50 mg dosage, it is approximately $6,720.
Where to Buy Tucatinib with a competitive price or cost
Get the best-branded drug from Emergency Drug with the most reasonable pricing across the world. We provide home delivery services all over the world. So, why delay in purchasing your essential medicine? Our convenient home delivery service ensures that you can access your medications from the comfort of your home, regardless of your location. Don’t hesitate; take advantage of our competitive pricing and reliable delivery services to meet your medical needs conveniently. Trust Emergency Drug to provide you with the medication you need, when you need it, delivered right to your doorstep.
Suggestion
Do not take any medicine without your doctor’s advice. Because it can be harmful to your body. So before taking any medicine defiantly consult with the doctor.
Worldwide Delivery Facilities
Andorra, Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe, Afghanistan, Albania, Algeria, American Samoa, Angola, Anguilla, Antarctica, Antigua & Barbuda, Armenia, Aruba, Bahamas, Barbados, Belarus, Belgium, Belize, Benin, Bermuda, Bhutan, Bolivia, Bosnia & Herzegovina, Botswana, Bouvet Island, British Virgin Islands, Brunei, Burkina Faso, Burundi.
Cameroon, Cape Verde, Cayman Islands, Central African Republic, Chad, China, Christmas Island, Comoros, Congo – Brazzaville, Congo – Kinshasa, Cook Islands, Czechia, Djibouti, Dominica, Ecuador, Egypt, El Salvador, Equatorial Guinea, Eritrea, Eswatini, Ethiopia, Falkland Islands, Faroe Islands, Fiji, French Guiana, French Polynesia, French Southern Territories, Gabon, Gambia, Gibraltar, Greenland, Grenada, Guadeloupe, Guam, Guernsey, Guinea, Guinea-Bissau, Guyana Haiti, Heard & McDonald Islands, Honduras, Hong Kong SAR China, Hungary, Iceland, Indonesia, Isle of Man, Jersey, Kazakhstan, Kiribati, Kyrgyzstan, Laos, Lesotho, Liberia, Liechtenstein, Luxembourg, China, Madagascar, Malaysia, Maldives, Mali, Malta, Marshall Islands, Martinique, Mauritania, Mauritius, Mayotte, Micronesia, Moldova, Monaco, Mongolia, Montserrat, Morocco, Mozambique, Myanmar (Burma), Namibia, Nauru, New Caledonia, Nicaragua, Niger, Niue.
Norfolk Island, North Macedonia, Northern Mariana Islands, Palau, Palestinian Territories, Panama, Papua New Guinea, Philippines, Pitcairn Islands, Portugal, Russia, Rwanda, Réunion, Samoa, San Marino, Senegal, Seychelles, Sierra Leone, Slovakia, Solomon Islands, Somalia, South Africa, South Georgia & South Sandwich Islands, South Korea, St. Helena, Suriname, Syria, Taiwan, Province of China, Tajikistan, Tanzania, Thailand, Timor-Leste, Togo, Tokelau, Tonga, Trinidad & Tobago, Tunisia, Turkey, Turkmenistan, Turks & Caicos Islands, Tuvalu, U.S. Outlying Islands, U.S. Virgin Islands, Uganda, Uruguay, Uzbekistan, Vanuatu, Vatican City, Vietnam, Wallis & Futuna, Western Sahara, Yemen and Zambia.
References
Revised: Jan 2023
2. tucatinib prescribing information
Issued: April 2020
Published: 12 Feb 2023
4. Conversations on Cancer: Living with Metastatic Breast Cancer
Published: 19 Oct 2023
Published: 2 Jul 2024
Note: The Generic’s information has been collected from various authentic sources and institutes.
For more Oncology medicine, visit our SHOP