Description
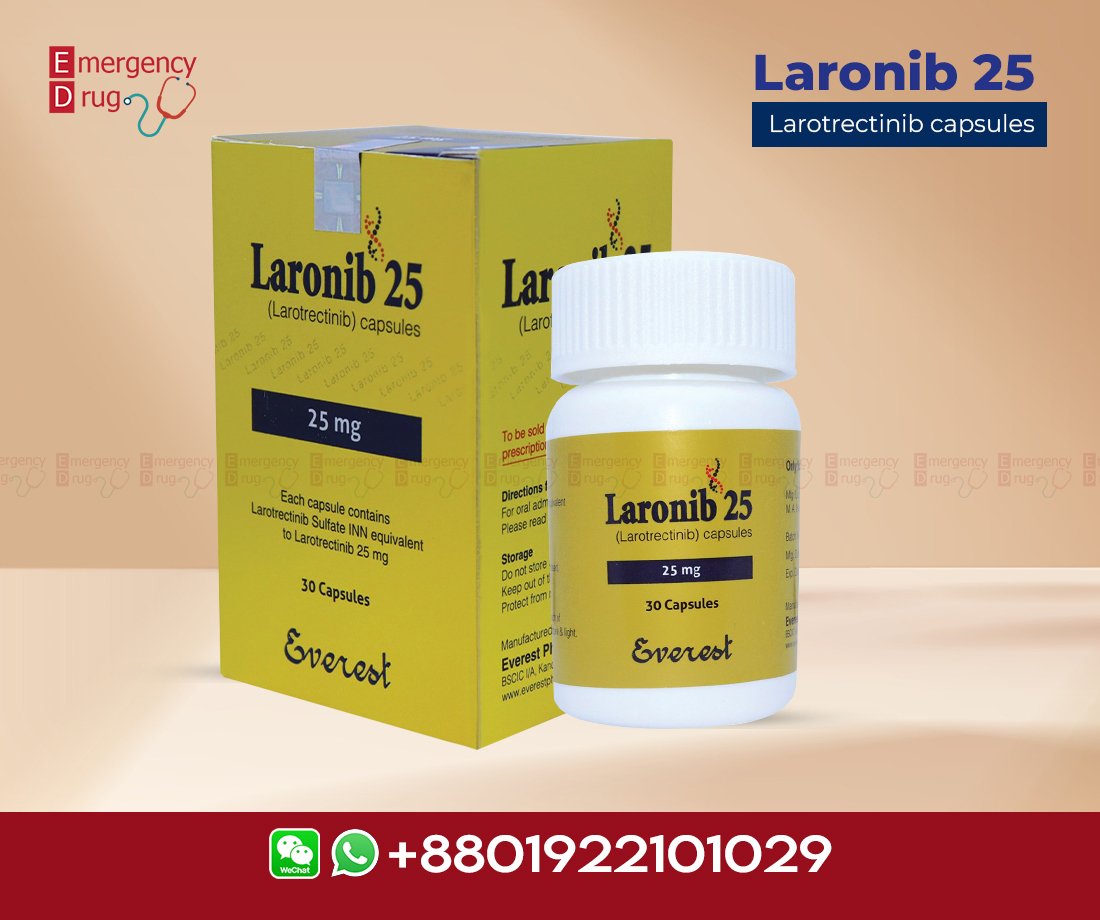
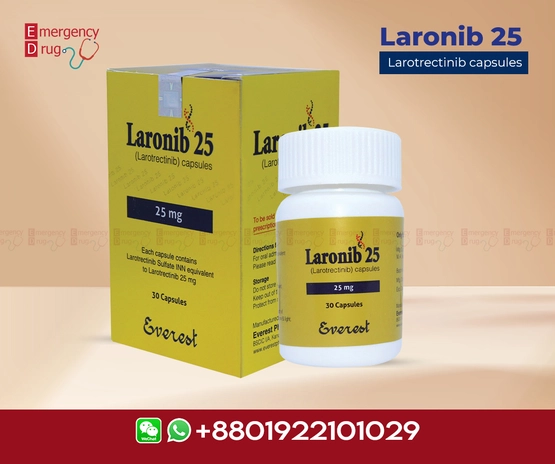
Laronib 25 mg (Larotrectinib), a novel oral inhibitor of TRK, is the first therapy approved for the treatment of adults and pediatric patients with solid tumors associated with an identified NTRK gene fusion. It is approved for patients with solid tumors and this biomarker. Those who have progressive metastatic disease that has no satisfactory alternative treatment. Because this tissue-agnostic drug has demonstrated durable responses and safety in this patient population.
Product Features
| Product Name | : | Laronib |
| Generic Name | : | Larotrectinib |
| Formulation | : | Capsule |
| Available Pack Size | : | 30’s Pot |
| Available Strength | : | 30 mg |
| Registrations | : | Export Only |
Solid Tumors With NTRK Mutation
The US Food and Drug Administration (FDA) approved larotrectinib (Vitrakvi; Loxo Oncology), the first drug approved for the treatment of adults and pediatric patients with solid tumors that have an NTRK gene fusion without a known acquired resistance mutation, are metastatic or where surgical resection is likely to result in severe morbidity, and have no satisfactory alternative treatments or that have progressed after treatment. Larotrectinib received accelerated approval based on overall response rate and duration of response data.
Mechanism of Action
Laronib is an inhibitor of TRK, including TRKA, TRKB, and TRKC.5 These kinases encoded by the genes NTRK1, NTRK2, and NTRK3. Using in vitro and in vivo tumor models, larotrectinib demonstrate antitumor activity in cells with constitutive activation of TRK proteins. Which resulted from gene fusions or the deletion of a protein regulatory domain, and in cells with TRK protein overexpression. Point mutations in the TRKC kinase domain (i.e., G623R, G696A, and F617L) result in resistance to larotrectinib.
Dosing And Administration
Patients should select for the consideration of larotrectinib treatment based on the presence of NTRK gene fusion in the tumor specimens. Currently no FDA-approved test is available for the detection of NTRK gene fusion. In clinical trials, the identification of positive NTRK gene fusion status determine prospectively in local laboratories using NGS or fluorescence in situ hybridization testing.
The recommended starting dose of Laronib in adults and young patients with a body surface area of ≥1 m2 is 100 mg orally twice daily, with or without food, until disease progression or unacceptable toxicity. For pediatric patients whose body surface area is <1 m2, the recommend starting dose of larotrectinib is <100 mg/m2 orally twice daily.
Laronib is available in a capsule form and as an oral solution (20 mg/mL). These dosage forms can use interchangeably. Larotrectinib capsules should swallow whole and should not chewed or crushed.
Drug Interactions
Laronib should not co-administer with strong cytochrome (CY) P3A4 inhibitors. If co-administration cannot avoid, the dose of larotrectinib should reduce by 50%. After the CYP3A4 inhibitor has discontinued for 3 to 5 elimination half-lives. The dose of larotrectinib that actually taken before the initiation of the CYP3A4 inhibitor can resume.
Warnings And Precautions
Neurotoxicity has reported with Laronib treatment. The majority of neurologic adverse reactions occur within the first 3 months of treatment (range, 1 day-2.2 years).
Modification of the Laronib dose require in patients who dizziness (3%), gait disturbance (1%), delirium (1%), memory impairment (1%), and tremor (1%). Larotrectinib treatment should withheld or permanently discontinue based on the severity of neurotoxicity.
Liver toxicity, including increased transaminases, has reported with larotrectinib treatment. Patients receiving larotrectinib should undergo liver test monitoring, including alanine transaminase and aspartate transaminase. Every 2 weeks during the first month of treatment, then monthly thereafter, and as clinically indicate for monitoring.
For more Oncology medicine, visit our SHOP




Reviews
There are no reviews yet.