Description
Product Features
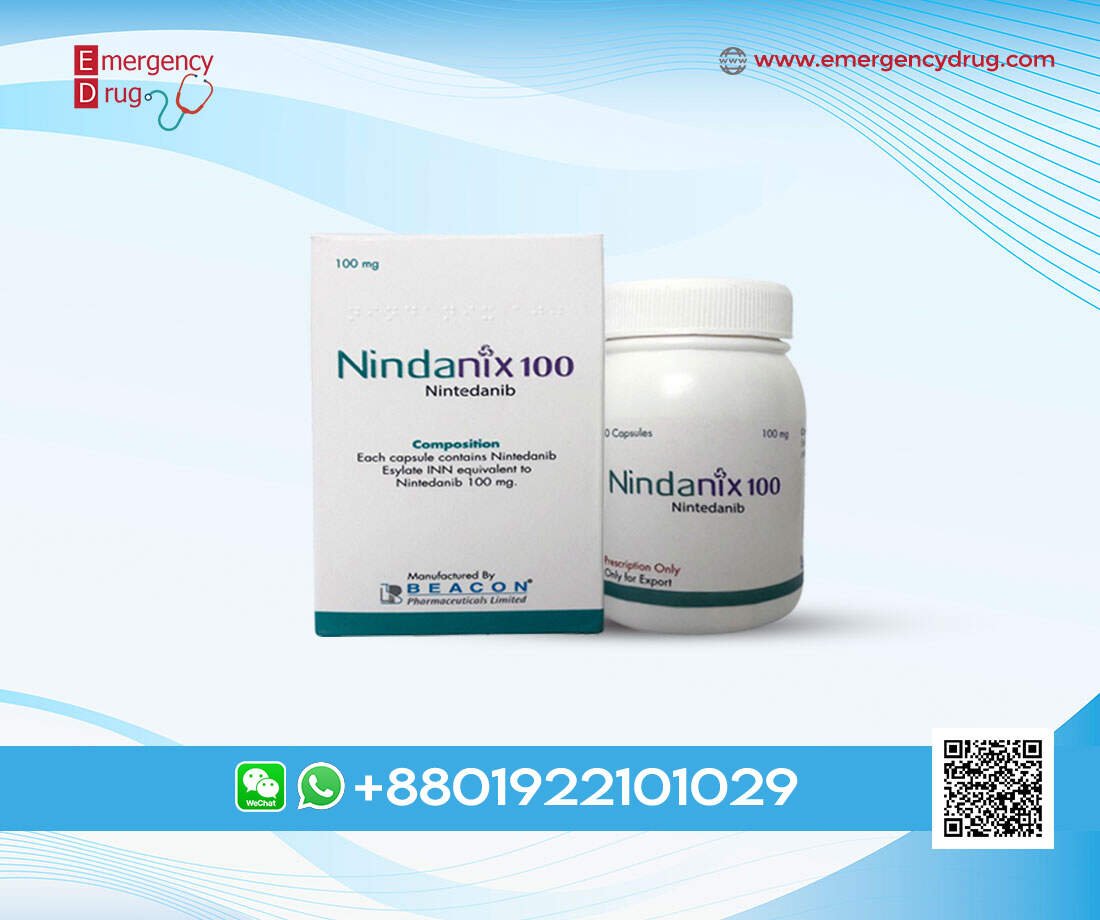
| Product Name | : | Nindanix |
| Generic Name | : | Nintedanib |
| Formulation | : | Capsule |
| Available Pack Size | : | 60’s Pot |
| Available Strength | : | 100 mg |
| Registrations | : | Export Only |
Manufacturer: Beacon Pharmaceuticals Limited
Nindanix 100 Capsule: Each capsule contains Nintedanib Esylate INN equivalent to Nintedanib 100 mg.
Therapeutic class: Anti Cancer
Nindanix is indicated for treating locally advanced, metastatic, or locally recurrent non-small cell lung cancer (NSCLC) adenocarcinoma type in combination with docetaxel for treating adult patients after the failure of first-line chemotherapy.
Clinical Pharmacology
Mechanism of Action
Nintedanib is a triple angiokinase inhibitor blocking vascular endothelial growth factor receptors (VEGFR 1-3), platelet-derived growth factor receptors (PDGFR α and ß) and fibroblast growth factor receptors (FGFR 1-3) kinase activity. Nintedanib binds competitively to the adenosine triphosphate (ATP) binding pocket of these receptors and blocks the intracellular signalling which is crucial for the proliferation and survival of endothelial as well as perivascular cells (pericytes and vascular smoothmuscle cells). In addition, Fms-like tyrosine-protein kinase (Flt)-3, lymphocyte-specific tyrosine-protein kinase (Lck) and proto-oncogene tyrosine-protein kinase Src (Src) are inhibited.
Indications
Nintedanib is kinase inhibitor indicated in combination with docetaxel for the treatment of adult patients with locally advanced, metastatic or locally recurrent non-small cell lung cancer (NSCLC) of adenocarcinoma tumour histology after first-line chemotherapy. Nintedanib is also indicated for the treatment of idiopathic pulmonary fibrosis (IPF).
Dosage and Administration
The recommended dose of Nintedanib is 200 mg twice daily administered approximately 12 hours apart, on days 2 to 21 of a standard 21-day Docetaxel treatment cycle. It must not be taken on the same day of Docetaxel chemotherapy administration (= day 1).
If a dose of Nintedanib is missed, administration should resume at the next scheduled time at the recommended dose. The individual daily doses of Nintedanib should not be increased beyond the recommended dose to make up for missed doses. The recommended maximum daily dose of 400 mg should not be exceeded. Patients may continue therapy with Nintedanib after discontinuation of docetaxel for as long as clinical benefit is observed or until unacceptable toxicity occurs.
Warning and Precautions
Hepatic impairment: Nintedanib is not recommended for use in patients with moderate or severe hepatic impairment. In patients with mild hepatic impairment (Child Pugh A), the recommended dosage is 100 mg twice daily approximately 12 hours apart taken with food. Consider treatment interruption, or discontinuation for management of adverse reactions in these patients.
Elevated liver enzymes and drug-induced liver injury: ALT, AST, and bilirubin elevations have occurred with Nintedanib, including cases of drug induced liver injury. In the post-marketing period, non-serious and serious cases of drug-induced liver injury, including severe liver injury with fatal outcome, have been reported. The majority of hepatic events occur within the first three months of treatment. Liver enzyme and bilirubin increases were reversible with dose modification or interruption in the majority of cases. Monitor ALT, AST, and bilirubin prior to initiation of treatment, at regular intervals during the first three months of treatment, and periodically thereafter or as clinically indicated. Temporary dosage reductions or discontinuations may be required.
Gastrointestinal disorders: Diarrhea, nausea, and vomiting have occurred with Nintedanib. Treat patients at first signs with adequate hydration and antidiarrheal medicine (e.g., loperamide) or anti-emetics. Discontinue Nintedanib if severe diarrhea, nausea, or vomiting persists despite symptomatic treatment.
Embryo-Fetal toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. Arterial thromboembolic events have been reported. Use caution when treating patients at higher cardiovascular risk including known coronary artery disease. Bleeding events have been reported. Use Nintedanib in patients with known bleeding risk only if anticipated benefit outweighs the potential risk. Gastrointestinal perforation has been reported. Use Nintedanib with caution when treating patients with recent abdominal surgery, previous history of diverticular disease or receiving concomitant corticosteroids or NSAIDs. Discontinue Nintedanib in patients who develop gastrointestinal perforation. Only use Nintedanib in patients with known risk of gastrointestinal perforation if the anticipated benefit outweighs the potential risk.
Side Effects
Very common side effects (may affect more than 1 in 10 people)
- Diarrhea
- Painful, numb and/or tingling feeling in fingers and toes (peripheral neuropathy)
- Feeling sick (nausea)
- Throwing up (vomiting)
- Pain in the stomach (abdomen)
- Bleeding
- Decrease in the number of white blood cells (neutropenia)
- Inflammation of the mucous membranes lining the digestive tract including sores and ulcers in the mouth (mucositis, including stomatitis)
- Rash
- Decreased appetite
- Electrolyte imbalance
- Increased liver enzyme values (alanine aminotransferase, aspartate aminotransferase, blood alkaline phosphatase) in the blood as seen from blood tests.
Common side effects (may affect up to 1 in 10 people)
- Blood poisoning (sepsis) – please see above
- Decrease in the number of white blood cells accompanied by fever (febrile neutropenia)
- Blood clots in the veins (venous thromboembolism)
- High blood pressure (hypertension)
- Fluid loss (dehydration)
- Abscesses
- Low platelet count (thrombocytopenia)
- Jaundice (hyperbilirubinaemia)
- Increased liver enzyme values (gamma-glutamyltransferase) in the blood as seen from blood
- Tests
- Weight loss
- Itching
Uncommon side effects (may affect up to 1 in 100 people)
- Occurrence of holes in the wall of your gut (gastrointestinal perforation)
- Serious liver problems
- Inflammation of the pancreas (pancreatitis)
- Myocardial infarction
- Renal failure
Use in Specific Populations
Pregnancy
Based on findings from animal studies and its mechanism of action Nintedanib can cause fetal harm when administered to a pregnant woman. There are no data on the use of Nintedanib during pregnancy. In animal studies of pregnant rats and rabbits treated during organogenesis, it caused embryo-fetal deaths and structural abnormalities at less than (rats) and approximately 5 times (rabbits) the maximum recommended human dose. Advise pregnant women of the potential risk to a fetus.
Lactation
There is no information on the presence of Nintedanib in human milk, the effects on the breast-fed infant or the effects on milk production. Nintedanib and/or its metabolites are present in the milk of lactating rats. Because of the potential for serious adverse reactions in nursing infants from it, advise women that breastfeeding is not recommended during treatment with Nintedanib.
Pediatric Use
The safety and efficacy of Nintedanib in pediatric patients has not been established.
Geriatric Use
Of the total number of subjects in phase 2 and 3 clinical studies of Nintedanib, 60.8% were 65 and over, while 16.3% were 75 and over. In phase 3 studies, no overall differences in effectiveness were observed between subjects who were 65 and over and younger subjects; no overall differences in safety were observed between subjects who were 65 and over or 75 and over and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Overdosage
There is no specific antidote or treatment for Nintedanib overdose. The highest single dose of Nintedanib administered in phase I studies was 450 mg once daily. In addition, 2 patients had an overdose of maximum 600 mg twice daily (b.i.d.) up to eight days. Observed adverse events were consistent with the known safety profile of Nintedanib, i.e. increased liver enzymes and gastrointestinal symptoms. Both patients recovered from these adverse reactions. In case of overdose, treatment should be interrupted and general supportive measures initiated as appropriate.
Storage condition: Store at below 30°C and dry place, away from light and moisture. Keep out of the reach of children.
To see more oncology medicine CLICK HERE




Alex Hickman –
They are the best! I always have a nice note from them in my shipment. Less costly than any place where I can shop locally. Thanks, and keep up the great work. Super fast shipment, and the product is always in wonderful condition.