Sofosbuvir for treatment of chronic hepatitis C
Mohammad Jewel
06 Jul, 2021
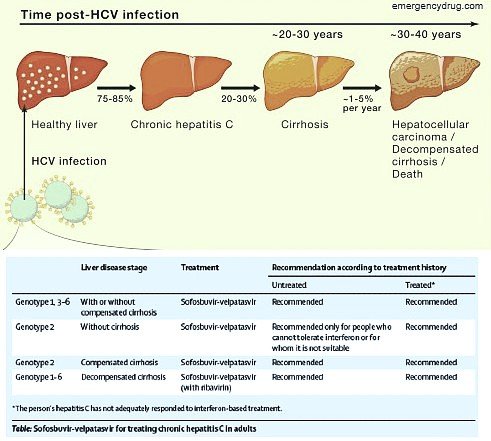
Table of Contents
Sofosbuvir for treatment of chronic hepatitis C
Chronic hepatitis C with Sofosbuvir has been a leap forward new drug for the treatment of patients with chronic hepatitis C. Sofosbuvir has various perfect properties, including pan genotypic movement, when every day dosing, no dinner confinements, couple of antagonistic impacts, insignificant medication tranquilize associations, high hereditary hindrance to opposition, great security and adequacy in patients with cutting edge liver ailment, and fantastic continued virologic reaction rates in patients with horrible standard qualities. In the new AASLD-IDSA hepatitis C rules, the mix of sofosbuvir in addition to peginterferon in addition to ribavirin is the suggested routine for patients with genotype 1,4,5, and 6 contamination.
Moreover, for patients ineligible to get interferon. Sofosbuvir in addition to simeprevir (with or without ribavirin) is prescribed, yet this mix isn’t a FDA-affirmed routine. For patients with genotype 2 or 3, the blend of sofosbuvir in addition to ribavirin is suggested. The utilization of sofosbuvir in mix with ribavirin gives the principal FDA affirmed all oral treatment for chronic hepatitis C. Of note, the action against genotype 3 seems not exactly with genotype 2 and treatment of genotype 3 disease requires a more drawn. Out all-oral course of treatment than with genotype 2. Sofosbuvir right now has a noteworthy job in the treatment of interminable HCV disease, however the remarkably surprising expense has filled in as a noteworthy obstruction for increasingly far reaching use and treatment of people with perpetual HCV contamination.
Class And Mechanism
Sofosbuvir (Sovaldi) is a nucleotide simple inhibitor of chronic hepatitis C infection NS5B polymerase—the key protein interceding HCV RNA replication. Sofosbuvir a prodrug and after ingestion it is quickly changed over to GS-331007, the overwhelming circling drug that represents more prominent than 90% of the fundamentally dynamic medication.
The compound GS-331007 proficiently taken up by hepatocytes, whereby cell kinases convert GS-331007 to its pharmacologically dynamic uridine simple 5′- triphosphate frame (GS-461203). This triphosphate compound impersonates the regular cell uridine nucleotide and fuse by the HCV RNA polymerase into the prolonging RNA groundwork strand, bringing about chain end. The dynamic shape GS-461203 focuses on the NS5B synergist site and goes about as a non-commit chain eliminator. The dynamic compound (GS-461203) does not repress DNA polymerases, RNA polymerases, or mitochondrial RNA polymerase.
Producer For United States
Gilead Sciences is the producer for sofosbuvir (Sovaldi) (Figure 1). The medication sofosbuvir was recently known as GS-7977 and was initially created by Pharmasset as compound PSI-7977. The medicine PSI-7977 find as the more dynamic diastereoisomer of the parent compound PSI-7851.
Cost And Medication Access
The discount procurement cost (WAC) for sofosbuvir is $1,000 per 400 mg pill. In like manner, the expense for the sofosbuvir segment in a 12-week treatment course is $84,000. (and the aggregate routine expense relies upon alternate prescriptions utilize in blend with sofosbuvir). For a 24-week course of sofosbuvir, the WAC is $168,000.
Gilead Sciences has a functioning sofosbuvir tolerant help program for qualify patients with chronic hepatitis C. who don’t have protection and not secure by Medicaid or Medicare. Data with respect to the Gilead Sciences sofosbuvir quiet help program can get at the Support Path for Solvaldi and Harvoni site and by reaching them specifically by telephone at 1-855-769-7284. (hours of activity Monday through Friday between 9:00 am and 8:00 pm Eastern Standard Time).
Unfavorable Effects
Sofosbuvir is commonly very much endured. The most widely recognized antagonistic impacts saw with sofosbuvir, when utilized in blend with ribavirin, have been weakness and migraine. Sofosbuvir is pregnancy classification B. To report speculated unfriendly responses, contact (1) Gilead Sciences, Inc. at 1-800-GILEAD-5 or (2) the FDA at 1-800-FDA-1088.
Key Drug Interactions
The real worry with medication communications exists with drugs that are solid inducers of intestinal P-gp. For example, rifampin and St. John’s wort, since these mixes may essentially bring down sofosbuvir and GS-331007 dimensions. The co-administration of sofosbuvir with the accompanying meds not to suggest in light of the fact. These drugs may altogether bring down sofosbuvir levels:
Anticonvulsants: carbamazepine, oxcarbazepine, phenobarbital, and phenytoin
Antimycobacterial: rifabutin, rifampin, rifapentine
Natural Supplements: St. John’s wort (Hypericum perforatum)
HIV Protease Inhibitor’s: tipranavir-ritonavir
For finish data on sofosbuvir-related medication communications, see the Drug Interactions area in the Sofosbuvir (Sovaldi) Prescribing Information.
Visit our Hepatitis c treatment Medicine product’s here:
