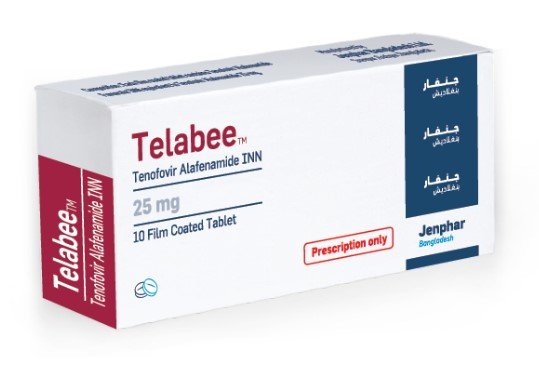
Description
Tenofovir Alafenamide (Tafecta) 25 mg indicate for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease.
Due to its enhanced stability in the plasma, Tafecta delivers tenofovir more efficiently to hepatocytes, with approximately 1/10 the dose of Tafecta Disoproxil. In addition, there is 89% less circulating in the plasma, resulting in reduced systemic exposure.
Product Features:
| Product Name | : Tafecta |
| Generic Name | : Tenofovir Alafenamide |
| Manufacturer | : Beacon Pharma Ltd |
| Indication | : Hepatitis B |
| Formulation | : Tablet |
| Strength | : 25 mg |
| Quantity | : 30 Tablets |
| Storage | : Below 30° |
| Registrations | : Export Only |
Mechanism Of Action
Tafecta 25 is a phosphonamidate prodrug of Tenofovir (2’-deoxyadenosine monophosphate analog). However, as a lipophilic cell-permeant compound enters primary hepatocytes by passive diffusion and by the hepatic uptake transporters OATP1B1 and OATP1B3. It is then converted to Tenofovir through hydrolysis primarily by Carboxylesterase 1 (CES1) in primary hepatocytes. Intracellular Tenofovir is subsequently phosphorylated by cellular kinases to the pharmacologically active metabolite Tenofovir Diphosphate. Tenofovir Diphosphate inhibits Hepatitis B Virus (HBV) replication through incorporation into viral DNA by the HBV reverse transcriptase, which results in DNA chain-termination. Tafecta Diphosphate is a weak inhibitor of mammalian DNA polymerases that include mitochondrial DNA polymerase γ and there is no evidence of toxicity to mitochondria in cell culture.
Pharmacodynamics
Cardiac Electrophysiology
In a thorough QT/QTc study in 48 healthy subjects, this medicine at the recommended dose or at a dose 5 times the recommended dose did not affect the QT/QTc interval and did not prolong the PR interval.
Pharmacokinetics
The pharmacokinetic properties of Tafecta are provided in Table.
Pharmacokinetic Properties of TENOFOVIR ALAFENAMIDE
| Absorption | TENOFOVIR ALAFENAMIDE |
| Tmax (h) | 0.48 |
| Effect of high fat meal (relative to fasting): AUClast Ratioa | 1.65 (1.51, 1.81) |
| Distribution | |
| % Bound to human plasma proteins | 80% |
| Source of protein binding data | Ex vivo |
| Blood-to-plasma ratio | 1.0 |
| Metabolism | |
| Metabolismb | CES1 (hepatocytes) Cathepsin A (PBMCs) CYP3A (minimal) |
| Elimination | |
| Major route of elimination | Metabolism ( > 80% of oral dose) |
| t½ (h)c | 0.51 |
| % Of dose excreted in urined | < 1 |
| % Of dose excreted in fecesd | 31.7 |
CES1 = carboxylesterase 1; PBMCs = peripheral blood mononuclear cells.
- Values refer to geometric mean ratio in AUClast [fed/fasted] and (90% confidence interval). High fat meal = ~800 kcal, 50% fat.
- In vivo, this is hydrolyzed within cells to form Tenofovir (major metabolite), which is phosphorylated to the active metabolite, Tenofovir Diphosphate. In vitro studies have shown that the Capsule is metabolized to Tenofovir by CES1 in hepatocytes, and by Cathepsin A in PBMCs and macrophages.
- t1/2 values refer to median terminal plasma half-life.
- Dosing in mass balance study: Tafecta 25 mg (single dose administration of [14 C] ).
Specific Populations
Geriatric Patients, Race, and Gender
No clinically relevant differences in Tenofovir Alafenamide or Tenofovir pharmacokinetics due to race or gender have been identified.
Patients with Renal Impairment
Relative to subjects with normal renal function (estimated creatinine clearance ≥90 mL/min), the Tenofovir Alafenamide and Tenofovir systemic exposures in subjects with severe renal impairment were 1.9-fold and 5.7-fold higher, respectively. The pharmacokinetics of this tablets have not been evaluated in patients with creatinine clearance less than 15 mL per minute.
Patients with Hepatic Impairment
Relative to subjects with normal hepatic function, medicine systemic exposures were 7.5% and 11% lower in subjects with mild hepatic impairment, respectively.
HIV and/or Hepatitis C Virus Co-infection.
The pharmacokinetics of Capsule not to fully evaluate in subjects coinfected with HIV and/or hepatitis C virus.
INDICATION Tenofovir Alafenamide
Tafecta indicate for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease.
DOSAGE AND ADMINISTRATION
Prior to initiation of Tenofovir Alafenamide, patient teste for HIV-1 infection. Once alone not t use in patients with HIV infection
It recommend that serum creatinine, serum phosphorous, estimated creatinine clearance, urine glucose, and urine protein be assessed before initiating Tafecta and during therapy in all patients as clinically appropriate.
Recommended Dosage in Adults
The recommended dosage of this tablets is 25 mg (one tablet) taken orally once daily with food.
Dosage in Patients with Renal Impairment
No dosage adjustment of this Capsule is required in patients with mild, moderate, or severe renal impairment. Tafecta is not recommended in patients with end stage renal disease (estimated creatinine clearance below 15 mL per minute.
Dosage in Patients with Hepatic Impairment
No dosage adjustment of f this capsule require in patients with mild hepatic impairment (Child-Pugh A). Tafecta not recommend in patients with decompensated (Child-Pugh B or C) hepatic impairment.
USE IN SPECIFIC Tenofovir Alafenamide
Pregnancy
There are no human data on the use of Tenofovir Alafenamide in pregnant women to inform a drug-associated risks of adverse fetal developmental outcome. In animal studies, no adverse developmental effects observe when it administer during the period of organogenesis at exposure equal to or 51 times (rats and rabbits, respectively) the tablets exposure at the recommended daily dose of Tafecta. No adverse effects observe in the offspring when TDF (Tenofovir Disoproxil Fumarate) administer through lactation at Tenofovir exposures of approximately 12 times the exposure at the recommended daily dosage of Tenofovir Alafenamide.
Lactation
It do not know whether Tenofovir Alafenamide and its metabolites are present in human breast milk, affect human milk production, or have effects on the breastfed infant. Tenofovir show to be present in the milk of lactating rats and rhesus monkeys after administration of Tenofovir Disproxil Fumarate.
Pediatric use
Safety and effectiveness of Tafecta in pediatric patients less than 18 years of age not to establish.
Geriatric Use
Clinical trials of Tenofovir Alafenamide did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Renal Impairment
No dosage adjustment of this tablets require in patients with mild, moderate, or severe renal impairment. It not to recommend in patients with end stage renal disease .
Hepatic Impairment
No dosage adjustment of this medicine require in patients with mild hepatic impairment (Child-Pugh A). The safety and efficacy of it tablets in patients with decompensated cirrhosis (Child-Pugh B or C) not t establish; therefore it not to recommend in patients with decompensate (Child-Pugh B or C) hepatic impairment.
For any inquiries please contact us
For more Oncology medicine, visit our SHOP





Reviews
There are no reviews yet.