Venetoclax
Venetoclax
Showing all 3 results
Venetoclax Price & Cost and Brands
Venetoclax is approved for the treatment of adult patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
It is approved for treating newly diagnosed acute myeloid leukemia (AML) in adults aged 75 years or older. It can also be used in patients who have other health problems that prevent them from safely using intensive chemotherapy. This medication is effective when combined with azacitidine, decitabine, or low-dose cytarabine.
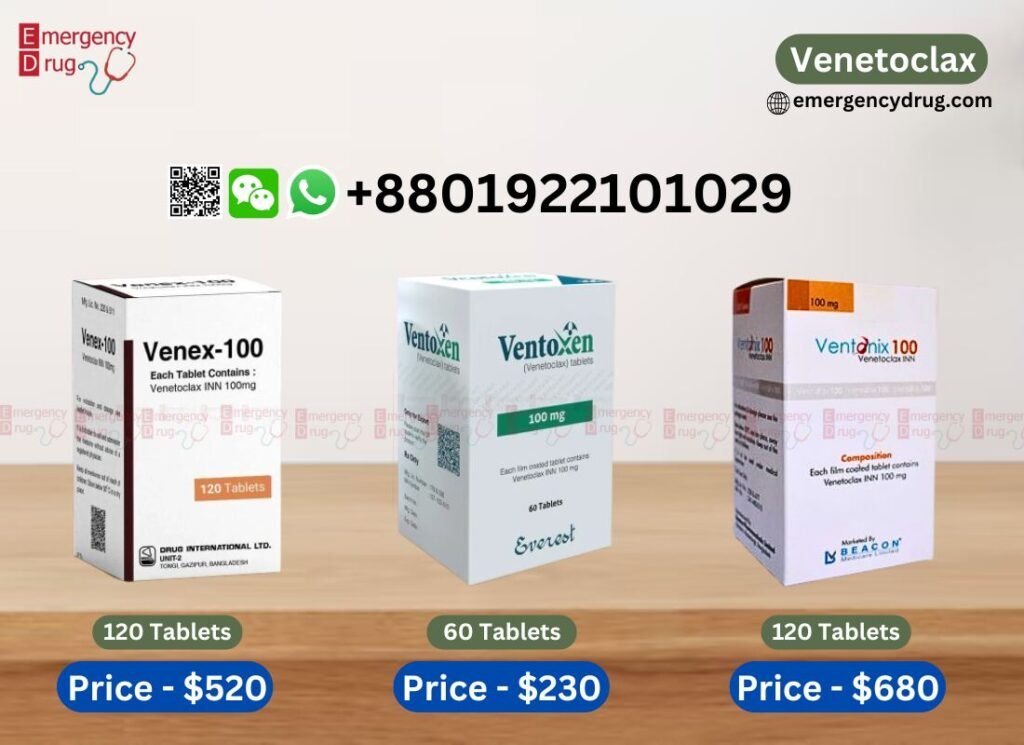
Available Brands and Venetoclax Price List
Brand Name |
Manufacture |
Quantity |
Venetoclax Price in USD |
| Venex 100 MG | Drug International |
120 Tablets |
$520 |
| Ventonix 100 MG | Beacon Pharma |
120 Tablets |
$680 |
| Ventoxen 100 MG | Everest Pharma |
60 Tablets |
$230 |
The Brand name of the Venetoclax Generic
The generic medicine Venetoclax is sold under the brand name Venclexta. It is a therapy primarily used for the treatment of chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML). Venclexta inhibits the BCL-2 protein, which is necessary for cancer cells to survive. By doing this, it helps to kill cancer cells.
Now most companies have been producing this generic with different brands’ names. Like, Beacon Pharmaceuticals Limited manufactures Ventonix, Everest Pharmaceuticals Ltd manufactures Ventoxen and Drug International Ltd manufactures Venex.
Venetoclax 100 mg
The 100 mg is a medicine taken by mouth, mainly used to treat chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML). It comes in tablet form and is usually taken once a day, with or without food, as directed by a doctor based on the patient’s condition and response to treatment. It is often used together with other treatments to enhance its effectiveness. Patients may need regular blood tests to monitor their health while taking this medication, as it can affect blood cell counts and increase the risk of infections.
Side Effects
To make sure it is safe for you, tell your doctor if you have ever had:
- Low Blood Cell Counts : This can lead to anemia, increased risk of infection, and bleeding issues.
- Nausea and Vomiting : Many affected people may experience gastrointestinal discomfort.
- Fever : Some may experience fever as a reaction to the medication.
- Diarrhea : This is a frequent side effect.
- Fatigue : Patients often report feeling unusually tired.
Mechanism of Action
- Venetoclax is a medicine that specifically blocks the B-cell lymphoma-2 (BCL-2) protein.
- BCL-2 is crucial for the survival of cancer cells, particularly in hematologic malignancies like chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML).
- Elevated levels of BCL-2 allow cancer cells to evade apoptosis (programmed cell death).
- By inhibiting BCL-2, It promotes the apoptosis of cancer cells.
- This action leads to a reduction in tumor size and improved patient outcomes, marking it as a significant advancement in blood cancer treatment.
Venetoclax FDA Approval
In 2015, the United States Food and Drug Administration (FDA) acknowledged the breakthrough potential of venetoclax for individuals with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who had relapsed, developed intolerance to, or were refractory to previous treatments.
Subsequently, in December 2016, venetoclax received approval for use in the European Union.
Clinical Trial
Venetoclax drug’s efficacy was established through a single-arm clinical trial involving 106 participants with CLL and a 17p deletion who had undergone at least one prior therapy.
Administered orally, the doses escalated from 20 mg to 400 mg over five weeks. The trial, spanning multiple countries including the US, Canada, France, Germany, Poland, the United Kingdom, and Australia, demonstrated that 80% of participants experienced either complete or partial cancer remission.
By June 2018, the FDA granted regular approval for venetoclax in CLL or SLL patients, regardless of 17p deletion status, who had undergone at least one prior therapy.
Then, in November 2018, the FDA approved venetoclax in combination with azacitidine, decitabine, or low-dose cytarabine for the treatment of newly diagnosed acute myeloid leukemia (AML) in adults aged 75 years or older, or those with comorbidities precluding intensive induction chemotherapy in the United States.
Venetoclax package insert
FAQ Section of Venetoclax Generic
1. How much does venetoclax cost?
Ans: The cost of Venetoclax typically ranges from $230 to $680 for a 30-day supply without insurance in the United States.
2. Who makes venetoclax?
Ans: The Generic is manufactured by AbbVie Inc.
3. Where is venetoclax made?
Ans: North Chicago, Illinois, USA
4. Is Venetoclax a cancer medicine?
Ans: Yes, It is a cancer medicine specifically used to treat certain types of blood cancers.
Feel Free You can Contact on WhatsApp or WeChat
Visit Our Shop for More Related Medicine