Subtotal: $225.00

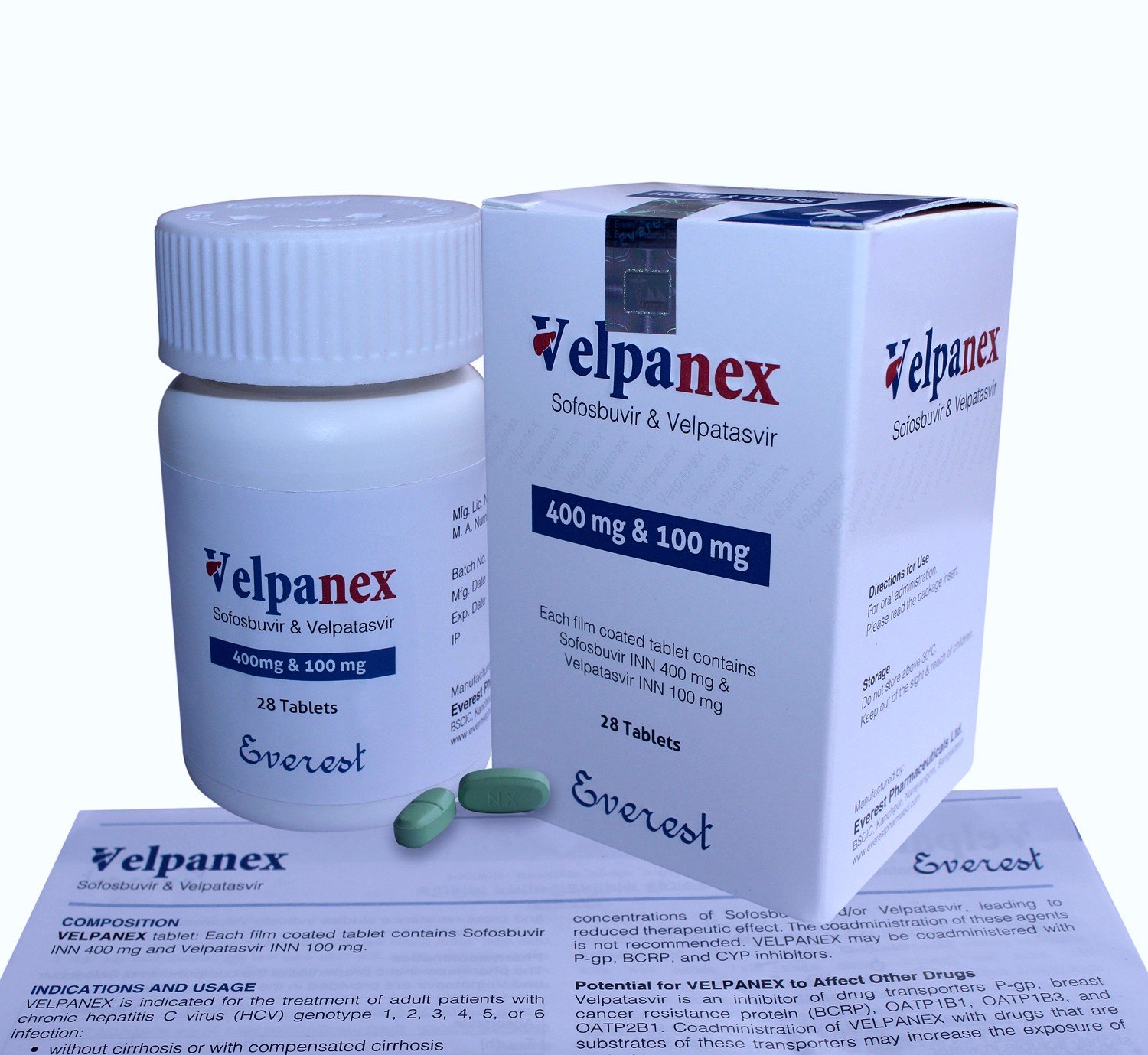
Sofosvel (Sofosbuvir+Velpatasvir) 400 MG
Price: $120.00
Out of stock
Sofosbuvir-velpatasvir is a pangenotypic NS5A-NS5B inhibitor single-pill combination regimen that has potent activity against hepatitis C virus (HCV) genotypes 1, 2, 3, 4, 5, and 6. It provides a much-needed option for patients with HCV genotype 3 infection, including those with compensated cirrhosis. Notably, an abbreviated duration of 8 weeks has not been studied with sofosbuvir-velpatasvir for any of the genotypes, except in conjunction with a third agent (voxilaprevir). Sofosbuvir-velpatasvir, like ledipasvir-sofosbuvir, may have have levels significantly reduced with acid-reducing agents, particularly proton-pump inhibitors.


Description
Sofosvel(Sofosbuvir Velpatasvir) is the global first generic oral tablet for pan-genotypic Chronic Hepatitis C treatment. This combination of drug has approved by US FDA & European Commission for treatment of patients with all genotypic Chronic Hepatitis C virus infection. Sofosvel use without cirrhosis or with compensated cirrhosis, and patients with advanced cirrhosis (Decompensated).
Sofosvel indicate for the treatment of adult patients with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5 or 6 infections
- With or without cirrhosis or with compensated cirrhosis
- With decompensated cirrhosis for use in combination with Ribavirin
Sofosbuvir+Velpatasvir is a direct-acting antiviral agent against the hepatitis C virus. It is an inhibitor of the HCV NS5B RNA-dependent RNA polymerase, which is essential for viral replication.
It indicate for the treatment of chronic hepatitis C (CHC) infection as a component of a combination antiviral treatment regimen.
Indications
Sofosbuvir and Velpatasvir combination is indicated for the prevention of adult patients with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5 or 6 infection-
- Without cirrhosis or with compensated cirrhosis
- With decompensated cirrhosis for use in combination with ribavirin
Therapeutic Class
Pharmacology
Dosage & Administration
The recommended dosage is one tablet (400 mg of Sofosbuvir and 100 mg of Velpatasvir) taken orally once daily. Recommended treatment regimen:
- Patients without cirrhosis and patients with compensated cirrhosis (Child-Pugh A): one tablet once daily for 12 weeks
- Patients with decompensated cirrhosis (Child-Pugh B or C): one tablet once daily and Ribavirin for 12 weeks. The recommended dosage of Ribavirin is based on bodyweight (1000 mg/day for patients < 75 kg and 1200 mg/day for ≥ 75 kg, in two divided dose/day)
Interaction
Drugs may decrease the concentrations of Sofosvel: Antacids, H2-receptor antagonists, Proton-pump inhibitors etc.
Coadministration is not recommended with: topotecan, Carbamazepine, Phenytoin, Phenobarbital, Oxcarbazepine, Rifabutin, Rifampin, Rifapentine, efavirenz, Tipranavir, Ritonavir, Hypericum perforatum.
Coadministration of Sofosbuvir and Velpatasvir combination, with Rosuvastatin, Atorvastatin may significantly increase the concentration of Rosuvastatin, Atorvastatin.
Contraindications
Side Effects
Pregnancy & Lactation
Precautions & Warnings
Use in Special Populations
Visit our another product here:

 Caboxen (Cabozantinib) 80 MG - 30 Capsules
Caboxen (Cabozantinib) 80 MG - 30 Capsules